Pakistan approves Chinese Sinopharm COVID-19 vaccine for emergency use
Share - WeChat

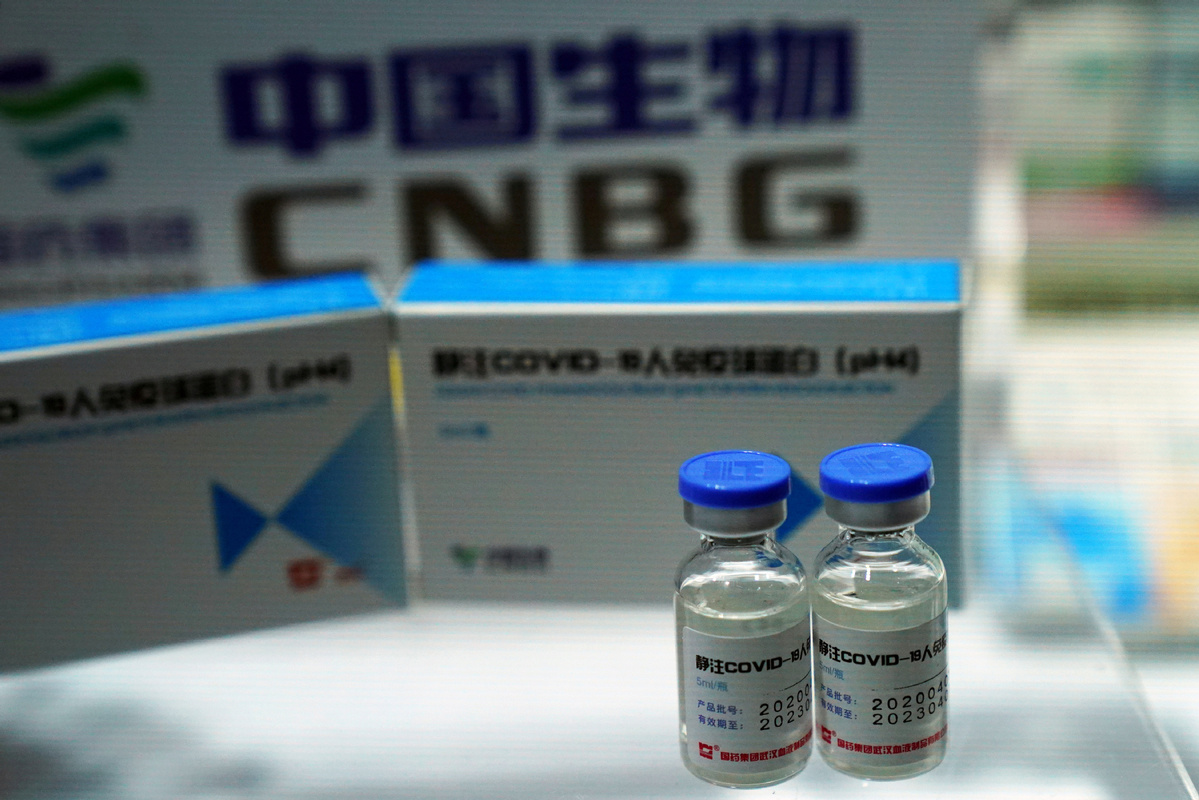
ISLAMABAD - Pakistan on Monday approved the Chinese Sinopharm COVID-19 vaccine for emergency use, a government statement said.
The Drug Regulatory Authority Pakistan (DRAP) said the vaccine, manufactured by China National Pharmaceutical Group (SinoPharm), had been given emergency use approval (EUA).
The authority had approved AstraZeneca's vaccine, developed with Oxford University, for emergency use at the weekend.
Pakistan's health ministry has said the country was in process of speaking to different vaccine candidates.
Reuters
Most Viewed in 24 Hours